Increasing profits and expanding product lines are two issues on the minds of many business owners in today's competitive world of screen printing. Breaking away from "price-only" competition involves a commitment to expanding your capabilities. Color-changing inks might be a great place to start.
You've seen color-change technology in a variety of forms: in the "mood rings" of the '70s, the stress testers of the '80s, and the forehead thermometers of the '90s. You may have seen printed garments that change color outdoors, and you may not even realize that those convenient on-package battery testers are screen-printed color-changing inks. Technology is advancing the use of these inks, leading to more and more interesting applications. Color-changing inks are not just used in novelty items anymore. They are rapidly becoming functional parts of manufactured industrial products in product labeling, the medical field, and security applications--and that may be just the beginning.
The two major groups of color-changing inks are thermochromic, which change color in response to temperature fluctuations, and photochromic, which respond to variations in exposure to UV light (primarily sunlight). Both materials are reversible and will change colors over and over again with the appropriate exposure. Other, emerging color-changing technologies include hydrochromics, which change in response to water, and piezochromics, which change color in response to pressure. Our discussion will be limited to thermochromics and photochromics, as they are the most widely used and most easily applied technologies available today.
Depending on the application, color-changing inks can be applied with a number of printing processes, including offset lithography, flexography, gravure, and screen printing. These are highly specialized inks that combine standard ink components with one of several color-changing agents, which will be described in the following sections. Since these inks are used on a wide variety of screen-printing substrates, it follows that they are offered in the typical solvent-based, water-based, plastisol, and UV formulations.
Thermochromics: Temperature-sensitive inks
The two types of thermochromic inks are liquid crystals and leucodyes. The most famous thermochromic application ever, the "mood ring," was a liquid crystal. Today, liquid crystals are used in many products, including aquarium thermometers, stress testers, and forehead thermometers. Unfortunately, liquid crystal thermochromics are very difficult to work with and require highly specialized printing and handling techniques. Because of these processing difficulties, we will limit our discussion to the other type of thermochromic ink, the leucodye.
Leucodye thermochromics are used in a wide range of applications because they add value in unique ways. Some of the applications include security printing, novelty stickers, product labels, advertising specialties, and textiles. Many of these applications go beyond novelty status, using thermochromic technology for functionality of the printed part. For example, in Figure 1, a leucodye thermochromic label indicates when the syrup is heated to the proper temperature.

Today, novelty applications aren't the only use for thermochromatic leucodye inks. As this example illustrates, the inks, which are colored when cold and clear when heated, can be used to produce temperature labels on food products that require warming before use. (All photos courtesy of Color Change Corp.)
In its cool state, a leucodye exhibits color, and when warmed, it turns clear or translucent. It takes a 5-10°F (3-6°C) shift to bring about a change in color, making leucodyes suitable for novelty items and general-purpose products not requiring distinct temperature readouts. For this reason, liquid crystal thermochromics, rather than leucodyes, are used in the production of thermometers.
Some products printed with leucodye thermochromic inks change from one color to another, rather than transitioning from colored to clear. This is achieved with an ink that combines a leucodye with a permanent-colored ink formulation. For example, the ink manufacturer may formulate a green ink by adding a blue leucodye to a yellow ink. In its cool state, the printed ink layer is green, and once warmed, reverts to yellow as the leucodye becomes clear or translucent. Leucodyes can be designed to change color at various temperature ranges, from as low as -13°F (-25°C) up to 150°F (66°C). A wide range of colors is also available.
Processing considerations for leucodye thermochromics
The types of thermochromic leucodye inks and general process considerations are shown in Table 1. In order to function, a leucodye requires a combination of chemicals working together in a system. This special system of materials needs to be protected from the components of the ink to which it is being added, so it is microencapsulated. The microencapsulation process takes a small droplet of the leucodye and coats a protective wall around it, as shown in Figure 2. The leucodye microcapsules contain the complete color-changing thermochromic system, which, when added to inks, give them their color-changing properties.
The microcapsules are also large. At 3-5 microns, they are at least ten times larger than the average pigment particle. Special considerations are usually involved in printing inks with these relatively large particles and include coarser screen mesh, heavier ink laydown, etc.

To prevent the leucodye chemicals from reacting with the inks in which they are used, the chemicals are encapsulated in a protective coating. A collection of leucodye microcapsules is shown here
Because the microencapsulation process cannot completely protect the leucodye system, ink manufacturers must take care to ensure compatibility with the inks to which they are added. This can involve slight modifications to standard ink formulations to make them more receptive to the addition of the leucodyes. So before adding any unapproved solvents or ink additives, you should confirm their compatibility by consulting with the thermochromic ink manufacturer.
Under normal conditions, thermochromic leucodye inks have a shelf life of six months or more. After they are printed, they function, or continue to change color, for years. The post-print functionality can, however, be adversely affected by UV light, temperatures in excess of 250°F (121°C), and aggressive solvents. Thermochromic textile inks will withstand about 20 washings before showing any significant deterioration and can last even longer when dried without heat. The use of chlorine bleach is not recommended, as this also will degrade the performance of the printed ink on textiles.
| Table 1 Thermochromic Leucodye Inks | ||||
| Thermochromatic leucodye inks incorporate large (3-5 micron) microcapsules of leucodye chemicals, and therefore require low mesh counts for printing. The leucodyes are available for all major ink types. | ||||
| Ink Type | Water base | UV | Epoxy | Plastisol |
| Substrates | paper, plastics | paper, plastics | glass, wood | textiles |
| Screen mesh | 110 thread/in. | 110-230 thread/in | 110 thread/in. | 110-305 thread/in. |
| Curing | air dry 1 hr | Expose approx. 40% longer than conventional UV ink | air dry 7-8 hrs | 325°F (use standard plastisol cure procedure) |
Photochromics: Light-sensitive inks
Photochromic inks change from clear when indoors to colored when taken outdoors. Specifically, they exhibit color in response to exposure to UV light from sunlight, black lights, or similar sources. UV light changes the chemical structure of the photochromic material and makes it absorb color like a dye. It then reverts to a clear state when the UV source is removed. The color change can occur thousands of times, depending on the application. A photochromic can also change from one color to another when it is combined with a permanent-pigment ink, similar to the leucodye-ink manufacturing process described earlier. These inks are also available in a full range of colors, including a four-color process system.
The most famous photochromic application is found in Transitions eyeglasses that darken in sunlight. Photochromics are also found in novelty applications such as ad specialties, stickers, and nail polish, and industrial applications that include security printing. Perhaps the most popular application is the color-changing T-shirt. An example is shown in Figure 3.
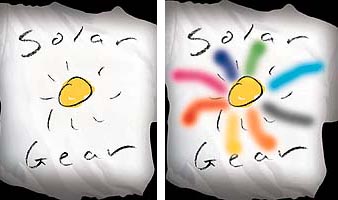
One of the most popular uses for photochromic inks is on screen-printed garments. As this design illustrates, the inks are transparent until exposed to a UV light source (sun, black light, etc.), at which time they take on their specific color characteristics. They return to a transparent state when UV exposure is discontinued
In their pure state, photochromics are powdered crystals that must be dissolved in the inks to which they are added. Some manufacturers microencapsulate the photochromics in their own system, as with leucodye microcapsules. Microencapsulating photochromic systems enables them to be used in inks that cannot dissolve them, such as water-based systems.
Even on cloudy days, photochromics exhibit bright color changes when taken outdoors. The color you see may differ slightly on very hot days or if a UV lamp, rather than sunlight, is used to excite the materials. These "quirks" in performance are a function of the unique materials that make up the photochromic system, and can be learned by most printers in a relatively short period of time.
Photochromic material is inherently unstable, and actually changes its chemical structure when exposed to UV light. In fact, the colored, or excited, form of a photochromic appears to be nearly broken in half, as shown in Figure 4. Because the dye is so vulnerable in its excited state, stabilization is the prime challenge for photochromic ink manufacturers. Without stabilization, most photochromic inks would not last even a few days in sunshine and may even expire before being printed. If shelf life is an important consideration, you should evaluate the stability of any photochromic ink before approving it for production.
The degradation of photochromic inks is more a function of UV exposure than the number of times it changes color. A properly stabilized photochromic ink will last for years on the shelf, but even the best of them will withstand only a few months of outdoor exposure after printing. The best photochromic textile inks will withstand about 20 washings after printing and are even more susceptible to the negative effects of chlorine bleach than their thermochromic counterparts. Bleach must never be used on garments printed with photochromic inks.
Photochromics are relatively new, having been introduced in the early 1990s, and their use has steadily increased as manufacturers have gained control over the stabilization process. A step up in this technology took place in 1995, leading to the most stable formulations to date. They are also available in flexographic inks, powders, and plastics.
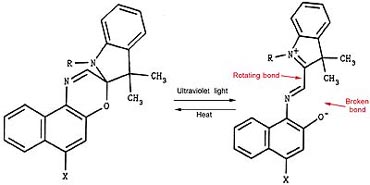
When photochromic crystals are exposed to a UV light source, it causes the inks to undergo a temporary chemical change in which the crystal molecules are nearly broken in half. When the UV source is removed, the molecules reform their original bonding structure
Processing considerations for photochromic inks
The types of photochromic inks and their general processing considerations are found in Table 2. These inks need to be printed on lightly colored surfaces, preferably white, to display the strongest color change. This is because photochromics are dyes, not pigments, and they absorb light. This absorption requires a light-colored background to reflect the remaining light so that the color-changing effect is maximized. A photochromic ink would still change color when printed on a black substrate, but you just wouldn't be able to see it. Normal inks use pigments, which reflect light, allowing their colors to be seen when printed over any colored substrate.
Because unexposed photochromic inks are usually invisible indoors, press setup can be more difficult than usual. The easiest solution is to have a black light at the press to excite the ink.
| Table 2 Photochromic Inks | |||
| Photochromic inks are available in all ink types except for UV formulations (UV curing could damage the UV-sensitive photochromic crystals). These crystals form a dye that is relatively transparent, so the inks should be used on lighter color garments and printed as thickly as possible. | |||
| Ink Type | Water base | Epoxy | Plastisol |
| Substrates | paper, plastics | glass, wood | textiles |
| Screen mesh | 110 thread/in. | 110 thread/in. | 110-305 thread/in. |
| Curing | air dry 1 hr | air dry 7-8 hrs | 325°F (use standard plastisol cure procedure) |
Ink manufacturers try to control the formulations so that little, if any, color is displayed indoors while achieving maximum color outdoors. This color control can be partially managed by the printer. A thinner ink laydown will affect lower indoor coloration, but will produce less intense outdoor coloration. A heavier ink laydown strengthens the colors both indoors and outdoors. Printers can test to find the balance that works best for them.
Economics of color-changing inks
Whether thermochromic or photochromic, color-changing inks contain highly specialized components that require extraordinarily careful manufacturing techniques. Not surprisingly, their per-gallon costs exceed that of traditional screen-printing inks. In fact, they are quite a bit more expensive. For example, a gallon of standard, UV-curable ink might cost $100-200, while a thermochromic UV ink will run about $800. Depending on the specific application and ink laydown, it typically costs between $0.005-0.02/sq in. of coverage of thermochromic UV ink.
Remember, however, that these inks impart a great deal more value to a printed substrate. The cost of the ink, as well as additional profit, can easily be incorporated into the price of the part. Take, for example, a gallon of photochromic plastisol ink, which costs four times the price of a regular plastisol. Normally, a square inch of coverage with a conventional plastisol costs about $0.0015. Depending on the specific design, a photochromic design may cost an additional $0.20 per garment. The good news is that the photochromic shirt exacts a $2-4 premium, simply because of the color-changing ink. That's adding value!
Color-changing materials offer fresh opportunities for additional profits and product line expansions. They are not difficult to work with and can be used in an ever-widening range of applications--many of which are still in the imaginations of some creative and enterprising screen printers. They are certainly worth a try.
About the author
Timothy J. Homola is the President and founder of Color Change Corporation, Addison, IL. The company, which was founded in 1992, specializes in color-changing materials, products, and technology. Homola has a bachelor of science degree in chemical engineering from the University of Illinois and a master's degree in management from the Kellogg School at Northwestern University.